Abstract
Introduction. Oncolytic Vesicular Stomatitis Virus expressing human interferon beta and the sodium iodide symporter reporter gene (VSV-hIFNβ-NIS) is in Phase 1 clinical testing for systemic administration in patients with relapsed multiple myeloma, acute myeloid leukemia and T cell lymphoma (NCT03017820). Local production of interferon β enhances virotherapy by activating the immune system, and restricts viral replication in normal cells by activating IFN induced antiviral responses. Thus, the virus propagates efficiently in IFN non-responsive cancer cells but is constrained in IFN responsive cells. Cancer cells that are defective in their viral sensing and IFN response pathways are exquisitely sensitive to VSV-IFNβ-NIS oncolysis, cumulating in tumor lysis syndrome and IFNβ induced toxicity in the MPC-11 mouse plasmacytoma model. Here, we evaluated the feasibility of using ruxolitinib, a clinically approved Jak1/2 inhibitor, to rescue mice from virotherapy induced TLS and IFNβ toxicity.
Methods. To characterize IFNβ toxicity in the absence of TLS , varying levels of mIFNβ were achieved in the blood of mice by intramuscular injection of an AAV1 vector encoding mIFNβ. For the virotherapy-associated TLS and IFNβ toxicity model, Balb/c mice with subcutaneous MPC-11 murine plasmacytoma were given one intravenous administration of VSV-mIFNβ-NIS. To determine whether the Jak/STAT inhibitor ruxolitonib could impact IFNβ toxicity, mice were treated with ruxolitinib (2 mg/dose by oral gavage) given twice a day for 10 days. All mice were monitored for adverse clinical signs andtumor size. Plasma IFNβ levels, CBC and comprehensive clinical chemistry panels were analyzed at necropsy.
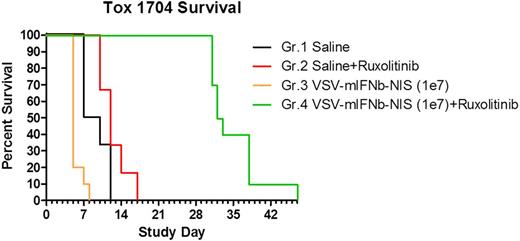
Results. There was a dose dependent increase in plasma IFNβ levels with increasing doses of AAV1-mIFNβ given intramuscularly to mice. Adverse clinical signs were seen in mice given high doses of AAV1-mIFNβ. There was a good correlation between IFNβ and AST or ALT levels. High circulating levels of IFNβ resulted in elevation in transaminases, thrombocytopenia, anemia and lymphopenia. IFN receptor defective mice were completely resistant to these toxic effects even with blood levels of >80,000 pg/ml IFNβ. Nontumor bearing Balb/c mice were shown to tolerate high IV doses of VSV-mIFNβ-NIS with transient lymphopenia and transaminases. In Balb/c mice with IFN nonresponsive MPC-11 tumors that support robust viral replication, VSV-mIFNβ-NIS therapy was highly effective for tumor control, but resulted in TLS and IFNβ toxicity, requiring early euthanasia of mice. Mice given VSV and a neutralizing anti-IFNR antibody did not show any adverse clinical signs due to IFNβ toxicity but control of tumor growth was significantly impaired in comparison to virus treatment alone. In contrast combination treatment of mice VSV and ruxolitinib resulted in reversal of lethal IFNβ toxicity without loss of effective tumor control.
Conclusions. Our data demonstrate that ruxolitinib reverses the toxicity of IFNβ seen following highly efficacious VSV-IFNβ-NIS virotherapy in the MPC-11 plasmacytoma model. Clinical evaluation of this promising combination is warranted.
Peng: Vyriad: Equity Ownership; Imanis Life Sciences: Employment, Equity Ownership. Russell: Vyriad: Equity Ownership; Imanis Life Sciences: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal